
Michael J. Denton
Biochemistry Department
University of Otago
Dunedin, New Zealand
One of the classic cases cited by Darwinists of supposed maladaptation in nature is the inverted design of the vertebrate retina.
In all non-vertebrate eyes, and in the pineal or dorsal eyes of primitive vertebrates, the photoreceptors point toward the light. However, in the vertebrate lateral eye, the photoreceptors point backwards away from the light towards the retinal epithelium and the choroidal blood sinuses. This arrangement necessitates the placement of the neural cell layer--which relays the visual image from the retina to the brain--between the photoreceptors and the light, and results in the blind spot where the axons of these neural cells leave the retina for the brain via the optic nerve.
Generations of Darwinists have seized on this apparently illogical arrangement and particularly the consequent “blind spot” as a case of maladaptation. The following comments by Dawkins are typical:1
Any engineer would naturally assume that the photocells would point towards the light, with their wires leading backwards towards the brain. He would laugh at any suggestion that the photocells might point away from the light, with their wires departing on the side nearest the light. Yet this is exactly what happens in all vertebrate eyes. Each photocell is, in effect, wired in backwards, with its wires sticking out on the side nearest to the light. This means that the light, instead of being granted an unrestricted passage to the photocells, has to pass through a forest of connecting wires, presumably suffering at least some attenuation and distortion (actually probably not much but, still, it is the principle of the thing that would offend any tidy-minded engineer!)
Vision is such an important adaptation in higher vertebrates that if the retina is indeed “wired wrongly” or “badly designed” it would certainly pose, as Dawkins implies, a considerable challenge to any teleological interpretation of nature.
However, consideration of the very high energy demands of the photoreceptor cells in the vertebrate retina suggests that rather than being a challenge to teleology the curious inverted design of the vertebrate retina may in fact represent a unique solution to the problem of providing the highly active photoreceptor cells of higher vertebrates with copious quantities of oxygen and nutrients.
The mammalian photoreceptor is capable of generating a measurable electrical response to a single photon of light--the minimal bundle of light energy. This remarkable capacity is dependent on a complex catalytic cascade consisting of a series of enzymes in the photoreceptor cell which massively amplifies the initial signal--the absorption by a single rhodopsin molecule of a single photon.2 This amplification process requires vast quantities of metabolic energy and consequently the photoreceptor layer has one of the highest metabolic rates of any known tissue.3 The oxygen consumption of the mammalian retina (per gram of tissue) is nearly 50% greater than that of the kidney, three times greater than the cerebral cortex and six times that of cardiac muscle.4 Moreover, because most of the metabolic activity of the retina is concentrated in the photoreceptor layer5--comprising less than half of the total mass of the retina--it is clear that the oxygen demands (per gram of tissue) of the photoreceptors are comparatively greater than such whole retinal estimates imply. Walls describes the photoreceptors in his classic The Vertebrate Eye as “greedy,”6 and greedy they are for both nutrients and oxygen (See Fig. 2). Indeed the high acuity and high sensitivity of the visual system in higher vertebrates is critically dependent on the very high metabolic rates of the photoreceptor cells.
The oxygen and nutrients for the voracious metabolic appetite of the photoreceptors are provided by a unique capillary bed, called the choriocapillaris, which is an anastomosing network of large and flattened capillaries which form a rich vascular layer situated immediately external to the photoreceptors,7 separated from them only by the retinal cell epithelial cell layer (RPE) and a special membrane--Bruch’s membrane--which together form a highly selective barrier which only allows passage into the retina of metabolites and nutrients required for the function of the RPE and photoreceptor cells. These capillaries are much larger than standard capillaries being between 18–50 microns in diameter.8 This unique network of blood channels gives every impression of being specially adapted to provide the photoreceptor layer with copious quantities of blood. Moreover, the lining of the capillaries is attenuated on the side closest to the photoreceptor cells--another indication that their fundamental purpose is the rapid and efficient delivery of nutrients to the photoreceptor layer. A beautiful illustration of this unique capillary bed, showing how it differs from a standard type capillary bed, is shown in Adler’s Physiology of the Eye (see Figure 1).9 Not only does the choriocapillaris give every appearance of being specially adapted for the provision of a copious supply of blood to the photoreceptors, it is a surprising and little-known fact that as much as 80% of the blood supply to the eye in mammals is carried through these remarkable choroidal vessels. (The retinal artery which enters the eye through the optic disc and supplies the neural layer--in front of the photoreceptor layer--carries only about 5% of the retinal blood supply.)10 As Walls puts it, “The blood filled tubing of the choroid exists solely in order to maintain a rich blood flow in the choriocapillaris and the latter exists solely to nourish the retina--with special reference to the greedy rod-cone layer.”11
 |
| Figure 1. The unique network of the choriocapillaris, which provides blood to the photoreceptors, as seen in a scanning electron micrograph of the cat choroid. Choroidal arteries (A) and veins (V) can be seen beneath the choriocapillaris. (After J.M. Risco and W. Noanitaya, Invest. Opthalmol. Vis. Sci. 19 [1980]:5) |
Because of the relatively massive blood flow through the choroidal capillary system, there is only a 3% difference in oxygen tension between the arterial and venous ends of the choroidal capillary system. This is virtually unique and contrasts with most capillary systems where the decrease may be up to 50%. In the case of the retinal system, for example, the decrease in oxygen content between the arterial and venous ends is given in some studies as 38%.12 As one authority puts it “Because of the great metabolic needs of the photoreceptors, the eye seems to have adopted the strategy of ‘swamping’ the choroid with blood to ensure that supply is never a problem.”13 In effect the photoreceptors are virtually bathed in arterial blood at all times. But remarkably, despite this very copious blood supply, oxygen tension drops across the photoreceptor from near arterial levels in the choroidal capillaries to close to zero near the inner segments, another indication of the very high metabolic demands of phototransduction.14, 15
The remarkable capacity of the unique choriocapillaris system to deliver copious quantities of oxygen to the photoreceptors has an important consequence--it obligates the necessity for a capillary network within the photoreceptor layer and this in turn allows the photoreceptor cells to be packed tightly together, thus maximizing the resolving power of the eye. It is also hard to imagine how a standard-type capillary network to carry the necessary quantities of blood directly through the photoreceptor cell layer could be arranged without causing at least some decrease in the packing density of the photoreceptors and a consequent decrease in the resolving power of the eye. (Interestingly, in all known high-acuity eyes, including the compound eyes of insects and other arthropods and the camera-type eyes of various groups, including the cephalopod and photoreceptors, are packed tightly together and not separated by either blood vessels or any other type of intervening tissues or structures.)
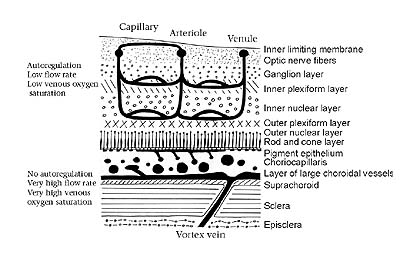 |
| Figure 2. Schematic representation of the vertebrate retina and its layers (light enters from the top of diagram). Note the intimate proximity of the choriocapillaris to the photoreceptors, which the choriocapillaris supplies with blood. (After Adler; Physiology of the Eye. [1987] C.V. Mosby Company. p. 185) |
Taken together, the evidence strongly supports the notion that the inverted retina and its major consequence (the positioning of the photoreceptors in the outer section of the retina where they are in intimate contact with the choriocapillaris) is a specific adaptation designed to deliver abundant quantities of oxygen to the photoreceptor cells commensurate with their high energy demands--especially in metabolically active groups such as the birds and mammals. Rather than being a case of maladaptation, the inverted retina is probably an essential element in the overall design of the vertebrate visual system.
This conclusion is reinforced by the difficulty of envisaging alternative means of delivering the required amounts of oxygen to the photoreceptor cell layer if the retina had the typical non-inverted design of the sort that might appeal to a “tidy-minded engineer.”
Blood absorbs light strongly, as witnessed by the fact that in the area centralis or macular region--which is the high-visual acuity part of the vertebrate retina--the density of the retinal arterioles and capillaries is often at a minimum or completely excluded. From this we can immediately discount one possible way of supplying the photoreceptors in a non-inverted retina where the photoreceptor would form the inner layer--pointing directly towards the light, i.e., by placing a choriocapillaris-type system of blood vessels in front of the photoreceptor cells, i.e., between the photoreceptors and the light. While such an arrangement might well deliver sufficient quantities of oxygen to the photoreceptors, the sensitivity and acuity of any such hypothetical “eye” would be greatly diminished by the highly absorbent complex of blood vessels positioned between the light and the photoreceptor layer.
Positioning a choriocapillaris or some equivalent system immediately behind the photoreceptor layer in a non-inverted retina would appear to be feasible alternative way of delivering oxygen to the photoreceptors but such an arrangement would place the retinal epithelium in a disadvantageous position to carry out its various functions involved in supporting and sustaining the photoreceptor cells including the phagocytosing and recycling the photoreceptor discs. In the inverted retina the retinal epithelium, situated between the choriocapillaris and the photoreceptors, is ideally placed to carry out these functions and to control the flow of metabolites, including vitamin A, to and from the choriocapillaris to the rods and cones. Indeed, in the inverted retina the retinal epithelium and the outer sections of the photoreceptor cells form a relatively isolated and distinct metabolic compartment sealed off on the inner side by the outer limiting membrane and on the outer side by the epithelium cells themselves. Also, in the inverted retina, the epithelium, positioned between the blood sinuses of the choroid and the retina, can function as part of the general blood-brain barrier controlling the flow of metabolites from the choroid into the inner regions of the retina. The need to maintain a blood-brain barrier may also mitigate against the hypothetical placement of a rich vascular bed between the photoreceptor layer and the neural retina.
The more deeply the design of the vertebrate retina is considered the more it appears that virtually every feature is necessary and that in redesigning from first principles an eye capable of the highest possible resolution (within the constraints imposed by the wavelength of light16) and of the highest possible sensitivity (capable of detecting an individual photon of light) we would end up recreating the vertebrate eye--complete with an inverted retina and a choriocapillaris separated from the photoreceptor layer by a supportive epithelium layer and so forth. (A more complete justification of this viewpoint is not possible here and is being prepared for publication elsewhere.)
Finally, there is the fascinating question of pre-adaptation. Although all vertebrates have the same inverted design the interesting question arises as to whether the inverted design is a necessity for high-resolution vision in the cold-blooded vertebrates such as fish which have lower metabolic rates than the warm-blooded vertebrates such as mammals and birds. In this context the high resolution eye of the cephalopods, including the octopus and squid, is instructive. The cephalopods have a typical non-inverted retina which is comparable in resolving power to the eyes of many vertebrates,17 have metabolic rates similar to that of fish and other cold-blooded vertebrates (even though the maximum oxygen capacity of cephalopod blood is only one third that of a fish),18 and inhabit an aquatic environment similar to that of many fish. This implies strongly that high-acuity vision in the eyes of cold-blooded vertebrates would be possible with a non-inverted retina and that it is only in the case of the higher and warm-blooded vertebrate species where the metabolic rates are far higher that the inverted arrangement to bring the photoreceptors adjacent to the choroidal vessels is a necessity for phototransduction. In other words, the inverted retinal design is almost certainly not an adaptive necessity in cold-blooded vertebrates.
It would seem that rather than being one of the classic “evidences” for undirected evolution and for maladaptation, the inversion of the retina is in fact highly problematic in terms of undirected models of evolution. Why on any undirected model should such an unlikely, improbable arrangement--unique in the animal kingdom--have appeared in the first place some 600 million years ago in the earliest of vertebrates who had presumably no need for high acuity vision and in all probability possessed photoreceptors with metabolic rates perhaps one or two orders of magnitude less than those of higher warm-blooded vertebrates today? If the non-inverted retina works so well for the cold-blooded cephalopods why did evolution go to such trouble to invert the retina in cold-blooded vertebrates? And is it really just fortuity that this curious event resulted in an adaptation which turned out to be essential for high acuity vision in the most advanced terrestrial vertebrates that appeared on earth long after this remarkable choice was made.
Rather than being a case of maladaptation, the inverted design of the vertebrate retina would seem to be a classic case of pre-adaptation--where an ancient adaptation was “chosen” long before its utility was of necessity. It is evidence for design and foresight in nature rather than evidence of chance. Evidently not all “tidy-minded engineers” get things right.
1. Dawkins, Richard (1986). The Blind Watchmaker. London: Penguin Books; pp. 93-94. return to text
2. Alberts, B., Bray, D., Lewis, R., Raff, M., Roberts, K., Watson, J.D. (1989). Molecular Biology of the Cell. New York: Garland Publishing, 1104-1108. return to text
3. Futterman, S. (1975). Metabolism and Photochemistry in the Retina, in Adler’s Physiology of the Eye, 6th edition, ed. R.A. Moses. St. Louis: C.V. Mosby Company, pp. 406-419; p. 406. return to text
4. Whikehart, D.R. (1994). Biochemistry of the Eye. Boston: Butterworth-Heinemann, p. 73. return to text
5. Futterman, op. cit., p. 407. return to text
6. Walls, G.L. (1963). The Vertebrate Eye. New York: Hafner Publishing Company; p. 652. return to text
7. Henkind, P., Hansen, R.I., Szalay, J. (1979). Ocular Circulation, in Physiology of the Human Eye and the Visual System, ed. R.R. Records. Maryland: Harper & Row Publishers, pp. 98-155; p. 119. return to text
8. Peyman, A.G., Sanders, D.R., Goldberg, M.F. (1987). Principles of Ophthalmology, Volume 1. Philadelphia: W.B. Saunders (First Indian edition, Jaypee Brothers, N. Delhi, India); p. 41. return to text
9. Alm, A. (1992). Ocular circulation, in Adler’s Physiology of the Eye, 9th edition, ed. W.M. Hart. St. Louis: C.V. Mosby Year Book; pp. 198-227; figure 6-7, p. 202. return to text
10. Henkind, op. cit., p. 140. return to text
11. Walls, op. cit. return to text
12. Alm, op. cit. return to text
13. McIlwain, T.J. (1996). An Introduction to the Biology of Vision. Cambridge: Cambridge University Press; see p. 14. return to text
14. Ibid., p. 71. return to text
15. Alm, op. cit.; fig. 6-17, p. 212. return to text
16. Denton, M.J. (1998). Nature’s Destiny. New York: The Free Press; see discussion in Chapter 3. return to text
17. Wells, M.J. (1962). Brain and Behavior in Cephalopods. Cambridge: Heinemann; p. 49. return to text
18. Ibid., pp. 152-153. return to text
Copyright © 1999 Michael J. Denton. All
rights reserved. International copyright secured.
File Date: 6.1.99