ATP Mechanisms Revealed
By Sean Henahan,
Access Excellence (not affiliated with ARN)
Baltimore, MD (9/15/98)- New X-ray crystallographic studies have revealed the working of adenosine triphosphate synthase, the basis of energy transport in all living organisms.
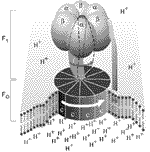
ATP captures the chemical energy released by the combustion of nutrients and transfers it to reactions that require energy, e.g. the building up of cell components, muscle contraction, transmission of nerve messages and many other functions. ATP synthase molecules located within mitochondria stick out on the mitochondria, attached to their inner surfaces in mushroom-like clusters. When food is broken down or metabolized for energy, the last stages of the process occur within the mitochondria.
The ATP synthase molecule, has two parts. Recently, scientists in Japan discovered that one part, the "mushroom stem," apparently rotates within the "mushroom cap." Last year, a Nobel prize was awarded to the researcher (Paul Boyer, Ph.D., UCLA) who suggested that forming ATP was somehow tied to this rotation, and the prize was shared with another researcher (John Walker, Ph.D., Medical Research Council Laboratory, Cambridge, England) whose team laid out one of two possible structures for the "cap," which is believed to be short-lived.
In new research, researchers at Johns Hopkins University determined the other structure, believed to be the most common form, in living organisms. The ATP synthase "mushroom cap," they found, contains three identical areas, arranged like a coil, where ATP is made. Each area is occupied with a different stage in ATP production.
As the "stem" rotates, it creates a powerful internal shifting in each of the three coiled sections within the cap. This shifting provides the energy to cause chemical changes. At one site, the "ingredients" for ATP come together. At another site, they assemble as ATP, and at the third site, the rotation readies the fully formed ATP to pop off the synthase molecule, for use throughout the cell.
A team led by L. Mario Amzel, Ph.D., and Peter Pedersen, Ph.D. used X-ray crystallography to reveal the molecular structure of adenosine triphosphate synthase. Inside, the molecule whirls around several times a second while it triggers production of ATP.
"It's one of the most complex molecules ever revealed, almost six times larger than the blood molecule hemoglobin," says Pedersen. It's also, the researchers agree, one of the tiniest and most powerful motors ever identified.
The researchers captured the image of the ATP synthase cap while all of its sites were in some stage of making ATP, which is essential for the constant recycling of its precursors. Without this recycling, Pedersen says, "people would have to produce more than half their body weight in ATP every day to meet their energy needs."
Some scientists have speculated that the same "free radicals" that damage skin in aging or cause mutations also might damage mitochondria. "If ATP synthase is a site of free radical damage, that could explain why we become less energetic with age," Pedersen says. Now that we know the molecular structures, we can pinpoint damaged regions if they occur."
The research appears in the current issue of the Proceedings of the National Academy of Sciences.